 illustration from Live Science
illustration from Live Science
The Gene-Editing Revolution: A New Chapter in Biology
Imagine a future where inherited diseases are curable, where crops thrive despite climate change, and where personalized therapies combat complex illnesses like cancer with unprecedented precision. This vision, once confined to the pages of science fiction, is rapidly materializing thanks to a groundbreaking technology known as CRISPR-Cas9. Often simply called CRISPR, this powerful gene-editing tool has emerged as a true revolution in biology, promising to fundamentally reshape our relationship with the building blocks of life. Its potential applications span human health, agriculture, environmental conservation, and fundamental scientific discovery. The CRISPR era is upon us, and grasping its implications is vital as we navigate the transformative, and sometimes challenging, path towards editing the future of life itself.
CRISPR-Cas9 Explained: Precision Engineering at the Molecular Level
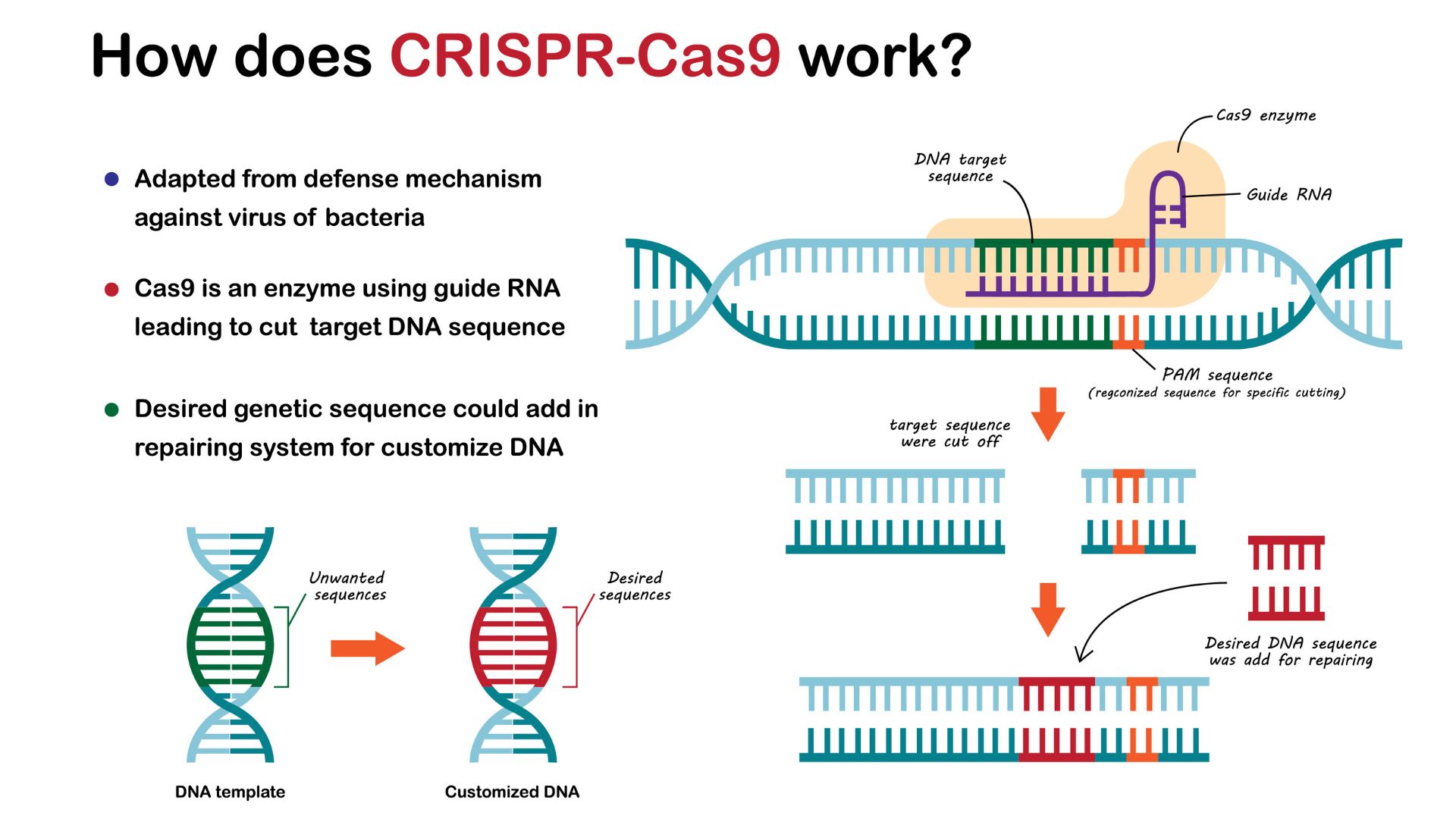
At its heart, CRISPR-Cas9 is a sophisticated molecular system borrowed from nature. Bacteria and archaea naturally use it as an immune defense to identify and destroy invading viruses by cutting their DNA. Scientists have ingeniously adapted this bacterial defense system into an incredibly versatile and precise instrument for editing genomes. Think of it as a highly accurate molecular scalpel capable of targeting and modifying specific locations within a DNA sequence.
The CRISPR-Cas9 system relies on two primary components:
- CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats): These are repeating segments of DNA found in bacteria, interspaced with unique 'spacer' sequences. These spacers are actually snippets of DNA from past viral infections, acting as a memory bank to recognize future threats.
- Cas9 (CRISPR-associated protein 9): This is an enzyme, a biological protein, that acts as the DNA-cutting machinery. It's the 'scissors' of the system.
Their collaboration is orchestrated by scientists using a specially engineered RNA molecule called a guide RNA (gRNA). This gRNA has a region that binds to the Cas9 enzyme and another region that is designed to match a specific target DNA sequence where an edit is desired. The gRNA acts as a molecular GPS, directing the Cas9 enzyme precisely to the intended site on the DNA. Once guided to the location, Cas9 makes a clean double-strand break in the DNA helix. This break activates the cell's natural DNA repair mechanisms, which scientists can then leverage in two main ways:
- Disrupting Gene Function: The cell's non-homologous end joining (NHEJ) repair pathway attempts to fix the break but is often error-prone. This can result in small insertions or deletions at the cut site, effectively disabling or 'knocking out' the gene. This is useful for turning off faulty or undesirable genes.
- Inserting or Correcting DNA: If a template DNA sequence containing the desired modification (like a corrected gene sequence) is introduced alongside the CRISPR-Cas9 system, the cell can use its homology-directed repair (HDR) pathway to accurately incorporate the new sequence or correct the existing one at the cut site.
This unparalleled ability to precisely cut and modify DNA has opened up transformative possibilities in biological research and potential medical treatments. Compared to older gene-editing methods, CRISPR-Cas9 is faster, more affordable, and significantly more accurate and efficient, cementing its status as a truly revolutionary tool.
From Benchtop to Bedside: CRISPR in the Medical Landscape
The most profound and immediate impact of CRISPR is unfolding in healthcare, particularly in the treatment of diseases with a genetic origin. Millions worldwide suffer from conditions stemming from errors or mutations in their DNA. CRISPR offers the unprecedented potential to directly address and correct these underlying genetic defects.
Treating Single-Gene Disorders
Diseases caused by a mutation in a single gene are prime candidates for CRISPR-based therapies. Conditions such as cystic fibrosis, sickle cell anemia, Huntington's disease, and certain forms of muscular dystrophy could potentially be treated, or even cured, by correcting the specific faulty gene responsible. Clinical trials are already progressing for several of these conditions. For example, researchers are using CRISPR to edit blood stem cells from patients with sickle cell disease to correct the mutation causing the disorder. These corrected cells can then be transplanted back into the patient, offering hope for a functional cure. Similarly, therapies for inherited forms of blindness are being developed, where CRISPR is delivered directly to the eye to fix the gene mutation in light-sensing cells.
A New Weapon Against Cancer
CRISPR is also proving incredibly promising in the fight against cancer. Its applications include:
- Engineering Immune Cells: T-cells, crucial components of the immune system, can be extracted from a cancer patient and genetically modified using CRISPR. This modification can make them better at recognizing and destroying cancer cells (e.g., by removing genetic 'brakes' that limit their activity or adding genes that help them specifically target tumor markers). These enhanced cells are then multiplied and reinfused, creating powerful personalized immunotherapy.
- Targeting Tumor Genetics: CRISPR could theoretically be used to directly edit genes within cancer cells that promote unchecked growth, or to disable genes that allow tumors to evade immune detection or resist chemotherapy.
Combating Infectious Diseases
Beyond genetic conditions and cancer, CRISPR is being explored for its potential against persistent infectious diseases. It could be used to:
- Remove Viral DNA: For viruses that integrate their genetic material into host cell DNA (like HIV or Hepatitis B), CRISPR could potentially be employed to cut out or inactivate the viral genome, potentially clearing the infection from the body.
- Create Cellular Resistance: CRISPR could modify host cells to make them inherently resistant to viral entry or replication, preventing infection altogether.
Accelerating Drug Discovery
CRISPR is an invaluable tool in the drug discovery process. Researchers can use it to quickly and precisely create cell lines or animal models with specific gene edits that mimic human diseases. This allows for a much deeper understanding of disease mechanisms and provides better systems for testing the efficacy and toxicity of potential new drug candidates, significantly speeding up research and enabling more targeted therapeutic development.
Beyond Human Health: CRISPR's Wide-Ranging Impact
The transformative power of the CRISPR revolution extends far beyond human medicine, holding the potential to reshape numerous other sectors.
Transforming Agriculture
CRISPR offers unparalleled precision in improving crops and livestock. Unlike older methods of genetic modification that often involved inserting genes from entirely different species, CRISPR allows for subtle, targeted edits to existing genes or the introduction of traits from closely related varieties. This precision engineering can lead to:
- Enhanced Yields and Nutrition: Editing genes to improve growth rates, increase nutrient uptake, or boost the production of beneficial compounds in plants.
- Natural Pest and Disease Resistance: Modifying plant genes to make them naturally immune to common pests and pathogens, reducing the need for environmentally harmful pesticides.
- Increased Environmental Resilience: Developing crops capable of withstanding adverse conditions like drought, high salinity, or extreme temperatures, which is increasingly critical in the face of climate change.
- Extended Freshness: Editing genes to slow down ripening or spoilage processes, reducing food waste.
Similar precise edits are being explored in livestock to improve animal health, productivity, and resistance to common diseases.
Fueling Fundamental Biological Research
CRISPR has quickly become an indispensable tool in basic biological research laboratories worldwide. Its relative ease of use allows scientists to rapidly and efficiently create genetic 'knockout' models (where a gene is inactivated) or 'knock-in' models (where a specific mutation or gene is introduced) in a wide variety of organisms, from bacteria and yeast to fruit flies, zebrafish, mice, and human cell lines. This capability allows researchers to dissect the function of individual genes, map complex biological pathways, and create accurate laboratory models of human diseases with unprecedented speed and scale.
Potential in Conservation
CRISPR is even being considered for use in conservation efforts. Potential, though often controversial, applications include:
- Controlling Invasive Species: Developing gene drives that could rapidly spread infertility genes through populations of invasive pests to control their numbers.
- Supporting Endangered Species: Introducing disease resistance into vulnerable populations or even exploring the deeply debated possibility of bringing back extinct species (de-extinction) by editing the genomes of their closest living relatives.
Navigating the Ethical Landscape: Challenges and Responsibility
With the immense power to edit life comes significant ethical responsibilities and complex challenges. The ability to modify the human genome, particularly in ways that could be passed down through generations, raises profound questions and concerns.
Distinguishing Somatic and Germline Editing
A critical distinction is made between two types of gene editing:
- Somatic Gene Editing: This involves modifying genes in non-reproductive cells (such as blood, muscle, brain, or skin cells). The changes are limited to the treated individual and are not inherited by their children. Many therapeutic applications aimed at treating existing diseases fall into this category. While requiring rigorous safety testing, this is generally considered less ethically complex than germline editing.
- Germline Gene Editing: This involves editing genes in reproductive cells (sperm or eggs) or in early embryos. The resulting genetic changes will be present in every cell of the developing individual and will be passed on to all future generations. This raises serious concerns about unintended genetic consequences for descendants, the potential for 'off-target' edits affecting other genes, and the potential for a 'slippery slope' towards using gene editing for non-medical enhancements (often referred to as 'designer babies').
Due to these profound ethical and safety concerns, most countries and leading scientific bodies currently prohibit or place strict moratoriums on human germline editing. The global scientific and ethical communities are engaged in active debate about whether, and under what circumstances, germline editing might ever be deemed acceptable, emphasizing the necessity for extensive public discourse and international consensus before any such applications are considered.
Safety and Off-Target Effects
Despite its remarkable precision, CRISPR is not without technical limitations. A significant challenge is the potential for 'off-target' edits – unintended cuts or modifications occurring at locations in the genome other than the intended target site. While ongoing research is developing methods to minimize this risk, it remains a critical safety consideration, especially in therapeutic applications. Ensuring the safe, efficient, and precise delivery of the CRISPR-Cas9 system to the correct cells and tissues within the body also presents a substantial technical hurdle.
Ensuring Accessibility and Equity
As CRISPR-based therapies move from clinical trials to approved treatments, ensuring equitable access will be paramount. These advanced medical interventions are likely to be very expensive, raising concerns about who will be able to afford them and the potential to widen existing global health disparities. Careful consideration of healthcare policies and international collaboration will be needed to ensure that the benefits of the CRISPR revolution are shared broadly and do not exacerbate inequalities.
The Edited Future: What Lies Ahead for CRISPR?
The field of CRISPR research is advancing at an extraordinary pace. Scientists are continuously refining the core technology, discovering and developing new CRISPR-associated enzymes (like Cas12 or Cas13, which targets RNA), and creating sophisticated variations that offer even greater precision or novel capabilities. These include techniques like base editing (which allows for changing a single DNA 'letter' without cutting both DNA strands) and prime editing (which enables highly precise targeted insertions, deletions, and all possible base changes). These 'next-generation' gene editors promise to overcome some of the limitations of the original CRISPR-Cas9 system.
We can anticipate several key developments in the coming years:
- Accelerated Clinical Progress: Expect to see a significant increase in clinical trials for a wider range of genetic diseases, various cancers, and infectious conditions, leading to the first wave of regulatory approvals and the availability of CRISPR-based therapies for patients.
- Improved Delivery Technologies: Intensive research is focused on developing safer and more efficient methods to deliver the CRISPR components to the specific target cells and tissues within the human body, using approaches such as engineered viral vectors, lipid nanoparticles, or physical delivery methods.
- Expanding Applications: CRISPR's utility will continue to grow beyond medicine into agriculture, industrial biotechnology, materials science, and environmental applications.
- Intensified Ethical and Regulatory Dialogue: Global discussions and debates surrounding the ethical implications of gene editing, particularly regarding potential germline applications, will continue and likely lead to the development of more detailed international guidelines and regulations.
- Deeper Biological Understanding: CRISPR will remain an essential tool for fundamental biological research, enabling scientists to unravel complex genetic interactions and gain unprecedented insights into the mechanisms of life.
The CRISPR revolution is not merely a scientific advancement; it is a societal transformation. It compels us to confront fundamental questions about health, disease, human identity, and our collective responsibility in shaping the biological future of our planet.
Conclusion: Wielding a Powerful Tool Responsibly
CRISPR-Cas9 is an exceptionally powerful and adaptable gene-editing tool that has already fundamentally changed biological research and holds immense potential to revolutionize medicine, agriculture, and numerous other fields. It offers profound hope for treating and potentially curing diseases that were previously considered intractable, improving food security for a growing global population, and unlocking deeper insights into the fundamental processes of life.
However, the power to edit life comes with significant ethical considerations and technical challenges that demand careful, thoughtful, and responsible navigation. Open dialogue among scientists, ethicists, policymakers, and the public, coupled with robust regulation and continued scientific rigor, is absolutely essential to ensure that this revolutionary technology is harnessed for the benefit of all humanity, minimizing potential risks while maximizing its positive impact.
The future is no longer just something that unfolds; with tools like CRISPR, it is increasingly something we are learning to actively edit. As this technology continues its rapid advancement, staying informed, engaging in critical thinking, and participating in the conversation about its development and application are more important than ever before.
Join the Conversation
What are your thoughts on the CRISPR revolution and its potential impact on our future? Share your perspective in the comments below!
Published on June 17, 2025
reference: Various Article on internet
Gema
Wordsmith and content writer passionate about creating high-quality content that informs, entertains, and inspires. Let me bring your brand's story to life.
All stories by : Gema

0 Comments